chlorine electron configuration|ground state electron configuration for chlorine : Manila Learn how to write the complete electron configuration of chlorine (Cl) using two different methods: orbit and orbital. Compare the rules, principles, and diagram. Time Changes in Chicago Over the Years Daylight Saving Time (DST) changes do not necessarily occur on the same date every year. Time zone changes for: Recent/upcoming years 2020 — 2029 2010 — 2019 2000 — 2009 1990 — 1999 1980 — 1989 1970 — 1979 1960 — 1969 1950 — 1959 1925 — 1949 1900 — 1924 1850 — 1899 .
PH0 · the electron configuration of potassium is 1s22s22p63s1
PH1 · noble gas configuration for chlorine
PH2 · ground state electron configuration for chlorine
PH3 · give the full electron configuration for sulfur
PH4 · electron configuration chart
PH5 · electron configuration calculator
PH6 · chlorine electron configuration unabbreviated
PH7 · chlorine electron configuration excited state
PH8 · Iba pa
Christmas fundraising ideas for schools. Santa Claus, presents and putting up the Christmas tree means one thing – it’s prime giving (and donating) time which makes fundraising easier for the kids. Here are some great Christmas ideas. #65: Secret Santa Sale. Get local companies to donate gifts to the secret Santa sale.
chlorine electron configuration*******Learn how to write the electron configuration for chlorine using the period table or an electron configuration chart. See the video and the step-by-step tutorial for writing the configuration notation.Learn how to write the electron configuration for argon (Ar) using the .Learn how to write the electron configuration notation for oxygen and .Learn how to write the electron configuration for sodium (Na) atom with .Learn how to write the complete electron configuration of chlorine (Cl) using two different methods: orbit and orbital. Compare the rules, principles, and diagram. Learn how to write the full and abbreviated ground state electron configuration of chlorine, a nonmetal with atomic number 17. See the table of sublevels, orbitals and electrons for each energy level.Learn the electron configuration of chlorine, a halogen with the symbol Cl and atomic number 17. Find out its properties, characteristics, history and more on this web page. Learn how to write the electron configuration for Chlorine (Cl) with 17 electrons using the electron configuration chart. Watch a step-by-step description and .Electron configuration The arrangements of electrons above the last (closed shell) noble gas. Melting point The temperature at which the solid–liquid phase change occurs. . Find the electron configuration of any element using this tool. Learn the rules, notation, and examples of electron configuration and valence electrons. Learn about the properties, reactions, and isotopes of chlorine, a halogen in group 17 and period 3. Chlorine has an electron configuration of [Ne]3s2 3p5 and forms .
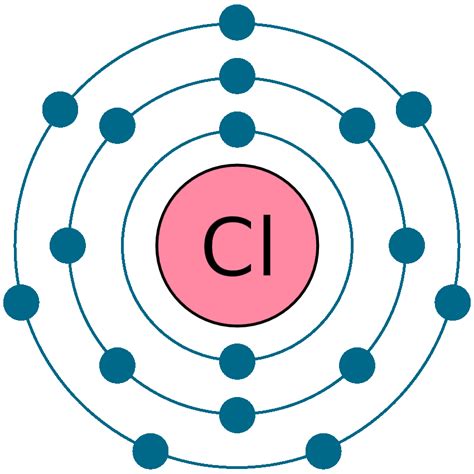
For writing the Chlorine Electron Configuration you first need to check the number of electrons for the Chlorine (Cl) atom (there are 17 electrons in chlorine atom). When you write the configuration .chlorine electron configuration Learn how to write the electron configuration of chlorine, a non-metal in group 17 of the periodic table. Find out the number of electrons, shells, and valence electrons in chlorine and its occurrence in .ground state electron configuration for chlorineThe electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons) are analogous in the electron configurations of their outer shells to their corresponding family members carbon, nitrogen, oxygen, fluorine, and neon, respectively, except that the principal . The electron configuration is the standard notation used to describe the electronic structure of an atom. Under the orbital approximation, we let each electron occupy an orbital, which can be .In this case, 2+2+6+2+6+2+10+6+2+1= 39 and Z=39, so the answer is correct. A slightly more complicated example is the electron configuration of bismuth (symbolized Bi, with Z = 83). The periodic table gives the .The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons) are analogous in the electron configurations of their outer shells to their corresponding family members carbon, nitrogen, oxygen, fluorine, and neon, respectively, except that the principal quantum . Inner transition elements are metallic elements in which the last electron added occupies an f orbital. They are shown in green in Figure 5.1.6 5.1. 6. The valence shells of the inner transition elements consist of the ( n – 2) f, the ( n – 1) d, and the ns subshells. There are two inner transition series:chlorine electron configuration ground state electron configuration for chlorineElectron configuration The arrangements of electrons above the last (closed shell) noble gas. Melting point The temperature at which the solid–liquid phase change occurs. Boiling point . Chlorine is what you might describe as a Jekyll and Hyde element; it is the friend of the synthetic chemist and has found a use in a number of 'nice .
A step-by-step description of how to write the electron configuration for Chlorine (Cl). In order to write the Cl electron configuration we first need to kn.
They are helium, neon, argon, krypton, xenon, and radon. A noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by the configuration of the remaining electrons. So for sodium, we make the substitution of [Ne] [ Ne] for the 1s22s22p6 1 s 2 2 s 2 2 p 6 part of the configuration.Chlorine is the second halogen, being a nonmetal in group 17 of the periodic table. Its properties are thus similar to fluorine, bromine, and iodine, and are largely intermediate between those of the first two. Chlorine has the electron configuration [Ne]3s 2 3p 5, with the seven electrons in the third and outermost shell acting as its valence . For writing the Chlorine Electron Configuration you first need to check the number of electrons for the Chlorine (Cl) atom (there are 17 electrons in chlorine atom).When you write the configuration you will have to put all 17 electrons of the chlorine atom in the orbitals that are around the nucleus of the Chlorine atom. The chemical symbol for the chlorine atom is Cl. The electronic configuration of chlorine is 1s 2 2s 2 2p 6 3s 2 3p 5. The distribution of electrons in the shell of the chlorine atom is 2, 8, 7. The .Assigning Electron Configuration . We write electronic configurations by following the aufbau principle (from German, meaning “building up”). First we determine the number of electrons in the atom; then we add .
2) The electron configuration of chlorine is: 1s 2 2s 2 2p 6 3s 2 3p 5. 3) What is the molecular geometry of the following? \(ClO_2\) -Bent or angular; the Cl is bonded to two ligands, has one lone pair and one unpaired electron. \(ClF_5\) -Square pyramid; the Cl is bonded to five ligands and has one lone pair. Add an electron to the anion electron configuration. For example, the ground state electronic configuration of chlorine is 1s²2s²2p⁶3s²3p⁵. For Cl −, it will be 1s²2s²2p⁶3s²3p⁶. Remove the outermost electrons in the cation, e.g. electron configuration for Mg 2+ will be 1s²2s²2p⁶.
A neutral chlorine atom has 17 electrons. Two electrons can go into the 1s subshell, 2 can go into the 2s subshell, and 6 can go into the 2p subshell. That leaves 7 electrons. Of those 7 electrons, 2 can go into the 3s subshell, and the remaining 5 electrons can go into the 3p subshell. Thus, the electron configuration of neutral chlorine atoms .

To write the orbital diagram for the Chlorine atom (Cl) first we need to write the electron configuration for just Cl. To do that we need to find the number. To write the orbital diagram for the Chlorine atom (Cl) first we need to write the electron configuration for just Cl. To do that we need to find the number.The electronic configuration of anions is assigned by adding electrons according to Aufbau's building up principle. We add electrons to fill the outermost orbital that is occupied, and then add more electrons to the next higher orbital. The neutral atom chlorine (Z=17), for instance has 17 electrons.Chlorine Electronic Configuration. Chlorine has an atomic number of 17. Therefore, its 17 electrons are distributed in the following manner: K shell – 2 electrons. L shell – 8 electrons. M shell – 7 electrons. The electron configuration of chlorine is illustrated below. It can be written as 1s 2 2s 2 2p 6 3s 2 3p 5 or as [Ne]3s 2 3p 5
Free gay porn videos, huge collection of free gay porno movies. New gay men twink sex porn tube vids delivered to you everyday only at BoyFriendTV.com
chlorine electron configuration|ground state electron configuration for chlorine